US/CAN 1-800-392-1353 | Outside US/CAN
Welcome to Care Oncology
Specializing in Metabolic Oncology
Since 2013 Care Oncology has been treating cancer patients, with multiple cancer types, using our patented COC Protocol™. The COC Protocol is a metabolic therapy that uses a combination of conventional pharmaceuticals which may work together to restrict the overall ability of cancer cells to take up and use (i.e., ‘metabolize’) energy.
Learn more about our Protocol and the Results we’ve achieved in our groundbreaking Metrics Study

What is Metabolic Oncology?
Cancer cells need sugar for replication. Metabolic oncology involves using a group of drugs called metabolic inhibitors. These metabolic inhibitors are intended to interrupt the production of energy in the cancer cells rendering them more vulnerable to other cancer fighting approaches.
Why Add the COC Protocol to Your Treatment & Post Treatment Plan
Current cancer treatments are designed to target cancer cells in the here and now, rather than dealing with the root causes that facilitated their development in the first place. Two of these root causes are metabolic dysfunction and chronic inflammation and paradoxically, some of the very treatments used to treat cancer may actually make these issues worse.
Today, in addition to targeting cancer metabolism through medication, our protocol has evolved to provide patients with nutrition and lifestyle recommendations to optimize your metabolic and inflammatory health. We’ll provide guidance around diet & nutrition, supplements, exercise, sleep and stress management, all of which play a key role in keeping you healthy.
The Benefits
The COC Protocol may:
- Improve your response to standard-of-care therapies (chemotherapy, immunotherapy, hormonal, and radiotherapies)
- Reduce side effects from your current cancer treatment
- Enable a process called apoptosis, or “programmed cell death,” of cancer cells
- Return your body back to a healthy metabolic and inflammatory state following cancer treatment to help minimize recurrence.
Program Features
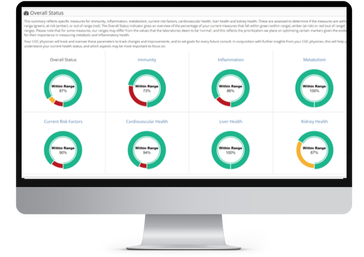
Results You Can Measure: While you’re enrolled in the program you’ll be able to visualize your progress through our new interactive digital platform (COC Health Insights) that shows you what you are doing well, where you’re at risk and what you need to improve.
Guided by Oncologists: Our program is delivered with oversight from experienced Metabolic Oncologists who can prescribe medications and other interventions to help improve your metabolic health.
Comprehensive Biomarker Testing: These tests look for specific metabolic and inflammatory markers that, if improved over time, lead to better health outcomes.
An In-Depth Health Report: A dynamic report informs you on your risk factors for cancer progression or recurrence based on your metabolic and immunologic functions. Quarterly check-ins will help us monitor and track your progress.
Protocol Medications: Care Oncology has a patented protocol which uses medications designed to target the specific energy requirements of cancer cells, disrupting their ability to grow and multiply.
Lifestyle Recommendations: Your Care Oncology oncologist will make lifestyle recommendations (diet, sleep, stress, exercise) to help improve your biomarkers and overall health.
Learn More About The COC Protocol and Our New Digital Platform, COC Health Insights™
Frequently Asked Questions
How to Reach Us
Please get in touch with us to discuss your individual situation further and to help you make an informed decision about the care that is best for you. You can either complete the contact form or schedule a call today to talk to a patient coordinator.
At Care Oncology, our expert oncologists provide safe, personalized guidance throughout your enrollment. This means you’ll get ongoing clinical support to address any questions or concerns you have while on the protocol.
Contact Us to Learn More
* By clicking on the “Submit” button, you consented Care Oncology to contact you via SMS messages, if needed.